Difference between revisions of "EnergyCrystals"
| Line 30: | Line 30: | ||
Pierre and Jacques Curie expanded upon Brewster’s experiments. They found that quartz and Rochelle salt display the strongest piezoelectricity, but cane sugar, topaz, and tourmaline also exhibit the effect. | Pierre and Jacques Curie expanded upon Brewster’s experiments. They found that quartz and Rochelle salt display the strongest piezoelectricity, but cane sugar, topaz, and tourmaline also exhibit the effect. | ||
| − | |||
| − | |||
== Make Rochelle Salt Crystals == | == Make Rochelle Salt Crystals == | ||
| Line 52: | Line 50: | ||
* Yes, Rochelle Salt Crystals have a very low melting point of about 75°C | * Yes, Rochelle Salt Crystals have a very low melting point of about 75°C | ||
* The low melting property can be used to easily make multi-crystalline materials | * The low melting property can be used to easily make multi-crystalline materials | ||
| − | + | * NaOH can be used instead of KOH | |
Revision as of 18:54, 5 December 2023
This page is to summarize our research in electro active crystal materials. There are many great instructions and descriptions online. The purpose of this summary is to give an overview of what we looked into and interesting insights we found. For detailed step by step instructions follow the links.
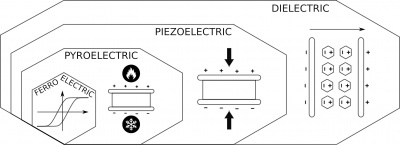
There are different electro active effects in crystal materials and those effects can be viewed in an onion hierarchy in some materials cumulate multiple effects.
Contents
Dielectric Effect
The most basic effect is the dielectric effect. When an electric field is applied by placing electrodes on two surfaces of crystals and applying an electric voltage, the crystal polarizes (dielectric polarisation). This means, while the crystals are electrically isolating materials and no current can flow through, in dielectric materials, charges can still shift slightly towards the poles.
What is this good for? Don't know, it's just the effect highest in hierarchy.
Piezoelectric Effect
Much more interesting is piezoelectric effect, the next in the hierarchy. All piezoelectric materials are also dielectric materials (while not all dielectric materials are also piezoelectric). A piezoelectric material has a special internal charge distribution such that when a mechanical force or vibration is applied it generates a polarization. So when the piezoelectric crystal with two conducive surfaces is put under mechanical stress it generates and electric voltage. This effect is reversible, so when an electric voltage is applied the crystal may deform.
Pyroelectric Effect
It get's even more fancy with the pyroelectric effect where a change in temperature can create an electric charge. And again, every pyroelectric crystal is also piezoelectric and dielectric.
We have not gone into this effect much yet.
pyroelectricity and piezoelectricity
A pyroelectric material generates a temporary voltage upon heating or cooling.
A piezoelectric material generates a voltage under the application of mechanical stress.
Sir David Brewster used Rochelle salt to demonstrate pyroelectricity and piezoelectricity in 1824.
Pierre and Jacques Curie expanded upon Brewster’s experiments. They found that quartz and Rochelle salt display the strongest piezoelectricity, but cane sugar, topaz, and tourmaline also exhibit the effect.
Make Rochelle Salt Crystals
Making crystals is easy and fun. An easy way to grow nice crystals is with Potassium alum. All ready the ancient Egyptians used it to make crystals. However while Potassium alum is dielectric, it is not piezoelectric.
An easy to grow crystal that shows all the amazing electro active properties is Rochelle Salt crystal. Also know as Sodium Potassium Tartrate Tetrahydrate, the crystals can be grown with common kitchen ingredients. All you need is washing soda (sodium carbonate), cream of tartar (potassium bitartrate or potassium hydrogen tartrate), and water. If you don't find washing soda you can make it from baking soda. See instructions blow.
Here just our learnings form spending hours of trying to make big and perfect crystals:
- While there are slightly different recipes, the ratio of water and cream of tartar should be somewhere between 1.25x and 1.38
- The ratio between cream of tartar and sodium carbonate is about 3.8x but this is not so important as you just add as much soda until it stops fizzling
- Yes, adding sodium slowly seems to be important, especially towards the end
- The solubility changes a lot with the temperature. So make sure you are at about 70°C
- The crystals start forming as the solution cools down. If the concentration is too high crystals form too quickly and you get just many small crystals
- If the concentration is too low the seed crystals dissolve before they start to grow. So getting the concentration right is important.
- Rochelle Salt crystals dissolve easily in water. So if you are not happy with what you get, a good way is to just dissolve the Rochelle Salt again and make it crystallize again.
- Seed crystals are an art on it's own. Suspend a perfectly formed seed crystal in the cooled down solution to grow big and perfect crystals.
- To attach seed crystals to a wire a good method we found is to heat a thin cooper wire and stick it into the crystal (by locally melting it)
- Yes, Rochelle Salt Crystals have a very low melting point of about 75°C
- The low melting property can be used to easily make multi-crystalline materials
- NaOH can be used instead of KOH
4.66g of KOH or 3.32g of NaOH
Melting Point: 75 °C Growing Potassium bitartrate $66 g / 100 mL Orthorhombic
It’s easy to make Rochelle salt using two common kitchen ingredients. Rochelle salt is sodium potassium tartrate tetrahydrate or potassium sodium tetrahydrate (KNaC4H4O6·4H2O). Rochelle salt yields large piezoelectric crystals, used for science experiments and as transducers in microphones and gramophone pickups
You need washing soda (sodium carbonate), cream of tartar (potassium bitartrate or potassium hydrogen tartrate), and water. If you can get washing soda, great! Use it. Most people don’t have ready access to washing soda, but can get baking soda (sodium bicarbonate). All you need to do to convert baking soda into washing soda is apply gentle heat.
Potassium bitartrate has a low solubility in water. It crystallizes in wine casks during the fermentation of grape juice, and can precipitate out of wine in bottles.
Links: https://www.instructables.com/Make-Rochelle-Salt/ https://sciencenotes.org/how-to-make-rochelle-salt-sodium-potassium-tartrate-tetrahydrate/
Sodium Potassium Tartrate Tetrahydrate
Growing Potassium bitartrate
It’s easy to make Rochelle salt using two common kitchen ingredients. Rochelle salt is sodium potassium tartrate tetrahydrate or potassium sodium tetrahydrate (KNaC4H4O6·4H2O). Rochelle salt yields large piezoelectric crystals, used for science experiments and as transducers in microphones and gramophone pickups
You need washing soda (sodium carbonate), cream of tartar (potassium bitartrate or potassium hydrogen tartrate), and water. If you can get washing soda, great! Use it. Most people don’t have ready access to washing soda, but can get baking soda (sodium bicarbonate). All you need to do to convert baking soda into washing soda is apply gentle heat.
Potassium bitartrate has a low solubility in water. It crystallizes in wine casks during the fermentation of grape juice, and can precipitate out of wine in bottles.
Links: https://www.instructables.com/Make-Rochelle-Salt/ https://sciencenotes.org/how-to-make-rochelle-salt-sodium-potassium-tartrate-tetrahydrate/